
Which of the following best describes what causes water molecules to exhibit properties such as cohesive and adhesive behavior?
a. water molecules are polar, having partially positive and partially negative ends.
b. water molecules have both a hydrophobic end and a hydrophilic end.
c. water molecules are capable of dissolving both polar and nonpolar substances.
d. water molecules have a high molecular weight, making them very dense.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1
You know the right answer?
Which of the following best describes what causes water molecules to exhibit properties such as cohe...
Questions






Biology, 01.08.2019 11:00


Mathematics, 01.08.2019 11:00



Mathematics, 01.08.2019 11:00

Mathematics, 01.08.2019 11:00

Biology, 01.08.2019 11:00







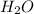 . It is a polar covalent molecule and in each O-H bond, oxygen has a partial negative charge and hydrogen a partial positive charge. Water molecules have strong cohesive forces as they can form intermolecular hydrogen bonds. Water molecules can also show strong adhesive forces with the walls of the container it holds due to the polar nature of the molecules.
. It is a polar covalent molecule and in each O-H bond, oxygen has a partial negative charge and hydrogen a partial positive charge. Water molecules have strong cohesive forces as they can form intermolecular hydrogen bonds. Water molecules can also show strong adhesive forces with the walls of the container it holds due to the polar nature of the molecules. 

