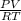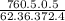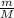A volatile organic liquid with known molecular weight is added to a vapor density flask which has a known volume. The flask is heated using a boiling water bath until all of the liquid has evaporated.
After heating for an additional 1 or 2 minutes, the flask is removed from the boiling water bath, corked, and cooled using a room temperature water bath. Predict the mass of condensed vapor.
Temperature of the hot water bath (ºC): 99.4
Volume of the flask (mL): 496.3
Molecular weight of the unknown (g/mol): 131.4
Barometer reading (mm Hg): 760.5
Calculate the mass of condensed vapor in g.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1
You know the right answer?
A volatile organic liquid with known molecular weight is added to a vapor density flask which has a...
Questions


Biology, 28.07.2019 18:00


History, 28.07.2019 18:00

Social Studies, 28.07.2019 18:00







Biology, 28.07.2019 18:00


Biology, 28.07.2019 18:00

History, 28.07.2019 18:00

English, 28.07.2019 18:00

Mathematics, 28.07.2019 18:00

Chemistry, 28.07.2019 18:00


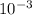 = 0.5 L
= 0.5 L