
Chemistry, 10.05.2020 07:57 lejeanjamespete1
What is the molecular formula of a compound with a molar mass of 92.001 g/mol and an empirical formula of NO2?
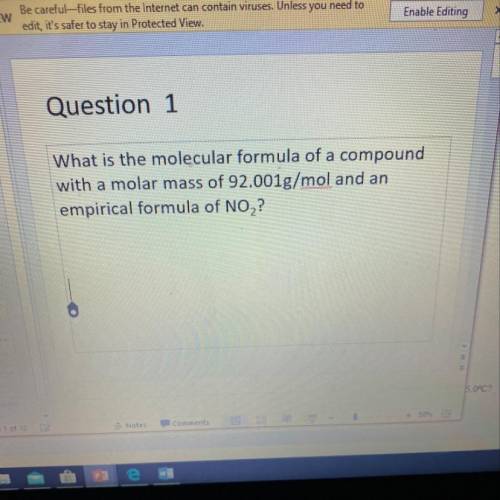

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
What is the molecular formula of a compound with a molar mass of 92.001 g/mol and an empirical formu...
Questions





History, 13.11.2019 14:31

History, 13.11.2019 14:31

Mathematics, 13.11.2019 14:31

Mathematics, 13.11.2019 14:31



History, 13.11.2019 14:31

Social Studies, 13.11.2019 14:31


History, 13.11.2019 14:31


English, 13.11.2019 14:31

Chemistry, 13.11.2019 14:31

History, 13.11.2019 14:31

Physics, 13.11.2019 14:31

Mathematics, 13.11.2019 14:31



