A ball has a volume of 6.35 liters and is at a temperature of
27.0°C. A pressure gauge attache...

Chemistry, 10.05.2020 06:57 ShugarLove4363
A ball has a volume of 6.35 liters and is at a temperature of
27.0°C. A pressure gauge attached to the ball reads 0.45
atmosphere. The atmospheric pressure is 1.00 atmosphere.
Calculate the absolute pressure inside the ball and the
amount of moles of air it contains. (First, find the absolute
pressure and then use that to find the moles using the ideal
gas law. Remember to use the correct ideal gas constant and
to convert from celsius to kelvin.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Questions


Physics, 18.06.2021 21:30


Mathematics, 18.06.2021 21:30

English, 18.06.2021 21:30

Mathematics, 18.06.2021 21:30



Mathematics, 18.06.2021 21:30

Mathematics, 18.06.2021 21:30

Mathematics, 18.06.2021 21:30

Biology, 18.06.2021 21:30

Mathematics, 18.06.2021 21:30


Arts, 18.06.2021 21:30


Mathematics, 18.06.2021 21:30

Mathematics, 18.06.2021 21:30


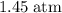 .Number of moles of air particles inside the ball, by the ideal gas law: approximately
.Number of moles of air particles inside the ball, by the ideal gas law: approximately 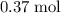 .
. 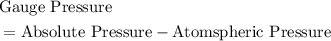 .
. .
.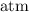 for pressure and
for pressure and 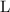 for volume.
for volume. .
.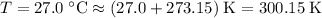 .
.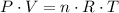 (after rearranging) to find the number of moles of gas particles in this ball:
(after rearranging) to find the number of moles of gas particles in this ball: .
.

