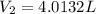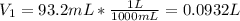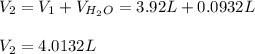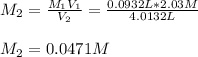is diluted with 3.92 L of water.


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
93.2 mL of a 2.03 M potassium fluoride (KF) solution
is diluted with 3.92 L of water.
is diluted with 3.92 L of water.
Questions

Social Studies, 22.03.2021 04:30

Mathematics, 22.03.2021 04:30


Mathematics, 22.03.2021 04:30


Mathematics, 22.03.2021 04:30


Mathematics, 22.03.2021 04:30


Mathematics, 22.03.2021 04:30


Mathematics, 22.03.2021 04:30

Mathematics, 22.03.2021 04:30




English, 22.03.2021 04:30



Social Studies, 22.03.2021 04:30