
Chemistry, 19.05.2020 03:01 kayla32213
Methane, CH 4 , burns in oxygen to give carbon dioxide and water according to the following equation:
CH 4 + 2 O 2 > CO 2 + 2 H 2 O
In one experiment, a mixture of 0.250 mol of methane was burned in 1.25 mol of oxygen in a sealed steel
vessel. Find the limiting reactant and excess reactants.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
Methane, CH 4 , burns in oxygen to give carbon dioxide and water according to the following equation...
Questions



Mathematics, 05.02.2021 20:30

Mathematics, 05.02.2021 20:30



Engineering, 05.02.2021 20:30


Mathematics, 05.02.2021 20:30

Mathematics, 05.02.2021 20:30


Advanced Placement (AP), 05.02.2021 20:30

Spanish, 05.02.2021 20:30

Advanced Placement (AP), 05.02.2021 20:30

Mathematics, 05.02.2021 20:30

Mathematics, 05.02.2021 20:30




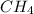 is the limiting reagent and
is the limiting reagent and 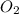 is the excess reagent.
is the excess reagent.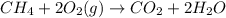
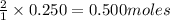 of
of 

