
Chemistry, 19.05.2020 15:58 cameronrandom00
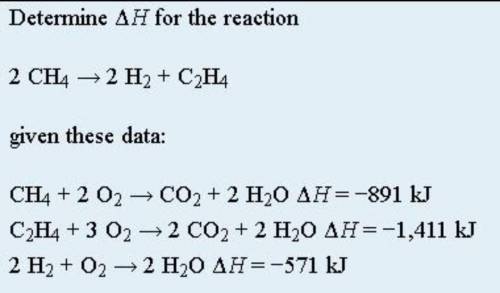
Hess’s law is very powerful. It allows us to combine equations to generate new chemical reactions whose enthalpy changes can be calculated, rather than directly measured. Besides that, Hess’s law states that when chemical equations are combined algebraically, their enthalpies can be combined in exactly the same way. Below points are to be taken as well: 1) If a chemical reaction is reversed, the sign on ΔH is changed; 2) If a multiple of a chemical reaction is taken, the same multiple of the ΔH is taken as well. As attached is an example of Hess Law problem solving question. By using Hess law , combine the equation algebraically and determine the enthaphy change of the reaction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Hess’s law is very powerful. It allows us to combine equations to generate new chemical reactions wh...
Questions


Mathematics, 10.04.2020 17:55

Mathematics, 10.04.2020 17:55

Biology, 10.04.2020 17:55







Mathematics, 10.04.2020 17:55






Mathematics, 10.04.2020 17:56






