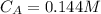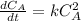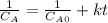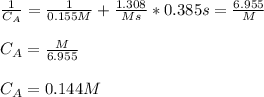Chemistry, 21.05.2020 00:16 Serenitybella
The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 1.308 M⁻¹ s⁻¹. For this reaction, the initial concentration of A is 0.155 M. What is the concentration of A after 0.385 seconds?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 1.308 M⁻...
Questions



Physics, 21.10.2019 20:30




Biology, 21.10.2019 20:30


Biology, 21.10.2019 20:30

Biology, 21.10.2019 20:30





Social Studies, 21.10.2019 20:30

Biology, 21.10.2019 20:30

Arts, 21.10.2019 20:30

Mathematics, 21.10.2019 20:30

Social Studies, 21.10.2019 20:30

Chemistry, 21.10.2019 20:30