
Chemistry, 21.05.2020 03:09 babygirl62716
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane and gaseous water in a 0.669 L flask at 1,020 K. At equilibrium, the flask contains 0.276 mol of CO gas, 0.207 mol of H2 gas, and 0.231 mol of methane. What is the water concentration at equilibrium (Kc = 0.30 for this process at 1,020 K)? Enter to 4 decimal places. HINT: Look at sample problem 17.7 in the 8th ed Silberberg book. Write a balanced chemical equation. Write the Kc expression. Calculate the equilibrium concentrations of all the species given (moles/liter). Put values into Kc expression, solve for the unknown.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane an...
Questions



Business, 28.11.2021 08:20

Mathematics, 28.11.2021 08:20


Biology, 28.11.2021 08:20

Mathematics, 28.11.2021 08:20


Social Studies, 28.11.2021 08:20

Mathematics, 28.11.2021 08:20


Mathematics, 28.11.2021 08:20

Chemistry, 28.11.2021 08:20

Physics, 28.11.2021 08:20

Mathematics, 28.11.2021 08:20

English, 28.11.2021 08:20

Mathematics, 28.11.2021 08:20



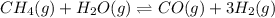
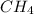 , [
, [ M = 0.345 M
M = 0.345 M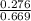 M = 0.413 M
M = 0.413 M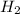 , [
, [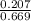 M = 0.309 M
M = 0.309 M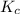 is expressed as:
is expressed as: ![K_{c}=\frac{[CO][H_{2}]^{3}}{[CH_{4}][H_{2}O]}](/tpl/images/0658/4057/27a18.png)
![\Rightarrow [H_{2}O]=\frac{[CO][H_{2}]^{3}}{[CH_{4}].K_{c}}=\frac{(0.413)\times (0.309)^{3}}{(0.345)\times (0.30)}= 0.1177](/tpl/images/0658/4057/4d76c.png)


