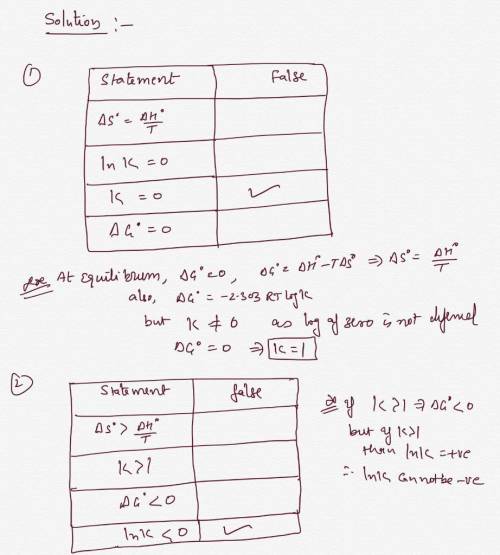
The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG Gibbs free energy of reaction and stands for the equilibrium constant. stands for the standard In each table, there may be one statement that is false because it contradicts the other three statements. If you find a false statement, check the box next to It. Otherwise, check the "no false statements box under the table. statement false? statement false? Ink>0
AH° R<1
AG'>0
AG'>0
In > AH">TAS
no false statements

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3


Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
The statements in the tables below are about two different chemical equilibria. The symbols have the...
Questions

Mathematics, 16.09.2021 06:30




Mathematics, 16.09.2021 06:30



Mathematics, 16.09.2021 06:30

Chemistry, 16.09.2021 06:30

Mathematics, 16.09.2021 06:30

Chemistry, 16.09.2021 06:30

Mathematics, 16.09.2021 06:30

Mathematics, 16.09.2021 06:30

Mathematics, 16.09.2021 06:30

Mathematics, 16.09.2021 06:30


Mathematics, 16.09.2021 06:30



History, 16.09.2021 06:30