
Chemistry, 20.05.2020 17:59 battlemarshmell
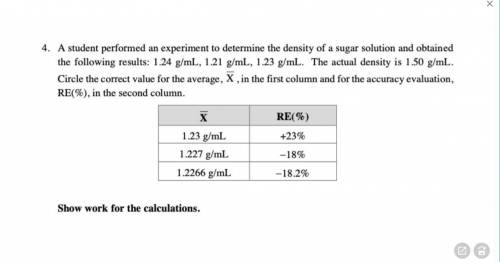
4. A student performed an experiment to determine the density of a sugar solution and obtainedthe following results: 1.24 g/mL, 1.21 g/mL, 1.23 g/mL. The actual density is 1.50 g/mL. Circle the correct value for the average, X, in the first column and for the accuracy evaluation, RE(%), in the second column.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

You know the right answer?
4. A student performed an experiment to determine the density of a sugar solution and obtainedthe fo...
Questions







Mathematics, 17.10.2020 14:01




Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01




Geography, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01


English, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01




