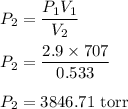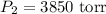Chemistry, 21.05.2020 23:58 washingtonisaia
A sample of chlorine gas occupies a volume of 707 ml at 2.90 atm. What is the new pressure in torr if the volume is compressed to 0.533 L?
a: 3,850 torr
b: 3.85 torr
c: 291 torr
d: 2,920 torr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1


Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
A sample of chlorine gas occupies a volume of 707 ml at 2.90 atm. What is the new pressure in torr i...
Questions

Biology, 17.07.2019 14:30

Biology, 17.07.2019 14:30


Biology, 17.07.2019 14:30

Mathematics, 17.07.2019 14:30

Biology, 17.07.2019 14:30

History, 17.07.2019 14:30

Mathematics, 17.07.2019 14:30

Social Studies, 17.07.2019 14:30

Social Studies, 17.07.2019 14:30





English, 17.07.2019 14:30

Social Studies, 17.07.2019 14:30

Biology, 17.07.2019 14:30


Mathematics, 17.07.2019 14:30

Biology, 17.07.2019 14:30

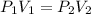
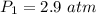
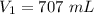
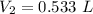
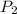 is the new pressure. So using Boyle's law we get :
is the new pressure. So using Boyle's law we get :