20 POINTS AND GIVING BRAINLIEST
The following reaction shows the products when sulfuric a...

Chemistry, 22.05.2020 01:05 baileyflemingde
20 POINTS AND GIVING BRAINLIEST
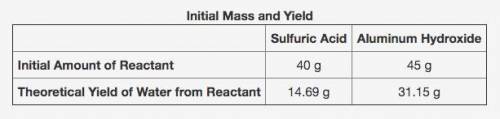
The following reaction shows the products when sulfuric acid and aluminum hydroxide react.
2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O
The table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. (See attached image)
What is the approximate amount of the leftover reactant?
A) 20.89 g of sulfuric acid
B) 22.44 g of sulfuric acid
C) 21.22 g of aluminum hydroxide
D) 23.78 g of aluminum hydroxide

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Questions

Mathematics, 12.07.2021 21:10


Mathematics, 12.07.2021 21:10

Chemistry, 12.07.2021 21:10


Mathematics, 12.07.2021 21:10




Mathematics, 12.07.2021 21:10

Mathematics, 12.07.2021 21:10

History, 12.07.2021 21:10

Mathematics, 12.07.2021 21:10


Social Studies, 12.07.2021 21:10




Mathematics, 12.07.2021 21:10



