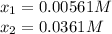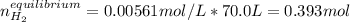Chemistry, 22.05.2020 02:10 Flameking1223
For the reaction
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calculate the number of moles of H2 that are present at equilibrium if a mixture of 0.680 mole of CO and 0.680 mole of H2O is heated to 700°C in a 70.0−L container.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3



Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
For the reaction
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calcu...
H2(g) + CO2(g) ⇆ H2O(g) + CO(g)
at 700°C, Kc = 0.534. Calcu...
Questions


Physics, 24.11.2020 21:50



Mathematics, 24.11.2020 21:50


Biology, 24.11.2020 21:50


Spanish, 24.11.2020 21:50


Biology, 24.11.2020 21:50


Mathematics, 24.11.2020 21:50

Mathematics, 24.11.2020 21:50





Mathematics, 24.11.2020 21:50

Mathematics, 24.11.2020 21:50

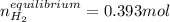
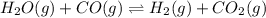
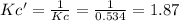
![Kc'=\frac{[H_2][CO_2]}{[H_2O][CO]}](/tpl/images/0660/7360/14ed3.png)
 due to the reaction extent:
due to the reaction extent:![Kc'=\frac{(x)(x)}{([H_2O]_0-x)([CO]_0-x)}=1.87](/tpl/images/0660/7360/2d81b.png)
![[H_2O]_0=[CO]_0=\frac{0.680mol}{70.0L}=0.0097M](/tpl/images/0660/7360/92f9f.png)