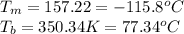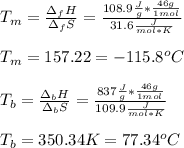Chemistry, 22.05.2020 01:10 questions61
For ethyl alcohol, C2H5OH, the enthalpy of fusion is 108.9 J/g, and the entropy of fusion is 31.6 J/mol •K. The enthalpy of vaporization at the boiling point is 837 J/g, and the molar entropy of vaporization is 109.9 J/mol •K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
For ethyl alcohol, C2H5OH, the enthalpy of fusion is 108.9 J/g, and the entropy of fusion is 31.6 J/...
Questions