2no(g) + o2 (g) --> 2no2
how many moles of o2 are required to produce 46.0 g no2?...

Chemistry, 30.08.2019 23:30 bchagnard2122
2no(g) + o2 (g) --> 2no2
how many moles of o2 are required to produce 46.0 g no2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Questions


History, 05.02.2021 07:20

History, 05.02.2021 07:20


Mathematics, 05.02.2021 07:20


History, 05.02.2021 07:20

Chemistry, 05.02.2021 07:20

Mathematics, 05.02.2021 07:20




Mathematics, 05.02.2021 07:20

Mathematics, 05.02.2021 07:20

History, 05.02.2021 07:20

Biology, 05.02.2021 07:20

Mathematics, 05.02.2021 07:20



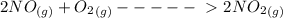
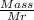

 mol
mol

