Benzene, a chemical used in the manufacture of many products, has a
density of 0.8765 g/cm3. W...

Chemistry, 22.05.2020 13:57 brandiwingard
Benzene, a chemical used in the manufacture of many products, has a
density of 0.8765 g/cm3. What would be the mass in mg of benzene if the
volume is 0.342 mL?
A. 89.9 mg
B. 390 mg
C. 2560 mg
D. 3340 mg
E. 300 mg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
Questions

History, 23.01.2022 21:00


Mathematics, 23.01.2022 21:00

Mathematics, 23.01.2022 21:00


Biology, 23.01.2022 21:10



History, 23.01.2022 21:10

Mathematics, 23.01.2022 21:10

History, 23.01.2022 21:10

Mathematics, 23.01.2022 21:10

Mathematics, 23.01.2022 21:10



English, 23.01.2022 21:10

Mathematics, 23.01.2022 21:10



Mathematics, 23.01.2022 21:10

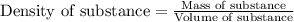
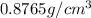
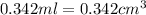
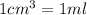
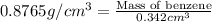
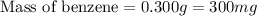 (1g=1000mg)
(1g=1000mg)

