
Chemistry, 22.05.2020 21:57 shanicar33500
(6) A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm.
What is the partial pressure of each gas?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

You know the right answer?
(6) A mixture of 40.0 g of oxygen and 40.0 g of helium has a total pressure of 0.900 atm.
What...
What...
Questions


Mathematics, 20.05.2021 03:50



English, 20.05.2021 03:50

Chemistry, 20.05.2021 03:50



Chemistry, 20.05.2021 03:50

History, 20.05.2021 03:50


Mathematics, 20.05.2021 03:50

History, 20.05.2021 03:50


Mathematics, 20.05.2021 03:50

Spanish, 20.05.2021 03:50


Mathematics, 20.05.2021 03:50


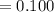 atm
atm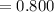 atm
atm 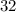 grams per mole
grams per mole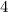 grams per mole
grams per mole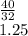
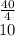
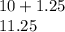
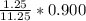 atm
atm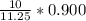 atm
atm

