
Chemistry, 22.05.2020 20:58 amelie1010
What would be the pH of an H2SO4 solution if the [H+] = 4.58 x 10-5 moles/liter?
9.66
4.34
5.67
NEXT QUESTION
3 ASK FOR HELP

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
What would be the pH of an H2SO4 solution if the [H+] = 4.58 x 10-5 moles/liter?
9.66
4....
9.66
4....
Questions


Biology, 03.08.2019 02:00

English, 03.08.2019 02:00


English, 03.08.2019 02:00




History, 03.08.2019 02:00





Mathematics, 03.08.2019 02:00


Mathematics, 03.08.2019 02:00


Health, 03.08.2019 02:00

Social Studies, 03.08.2019 02:00

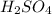 solution is 4.34
solution is 4.34![pH=-\log [H^+]](/tpl/images/0662/1654/37e81.png)
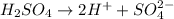
![pH=-\log[4.58\times 10^{-5}]](/tpl/images/0662/1654/0f1e2.png)
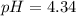
![[H^+]](/tpl/images/0662/1654/07acb.png) is
is ![4.58\times 10^{-5}]](/tpl/images/0662/1654/335c3.png) is 4.34
is 4.34

