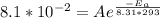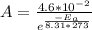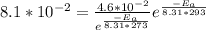Chemistry, 22.05.2020 00:04 caromaybelline71
A first order reaction has rate constants of 4.6 x 10-2 s-1 and 8.1 x 10-2 s-1 at 0ºC and 20ºC, respectively. What is the value for the activation energy?
A.
0.566 J/mol
B.
2.5 x 10-4 J/mol
C.
2260 J/mol
D.
18,800 J/mol
E.
1.76 J/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1


Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
A first order reaction has rate constants of 4.6 x 10-2 s-1 and 8.1 x 10-2 s-1 at 0ºC and 20ºC, resp...
Questions


Biology, 20.06.2021 14:00


Physics, 20.06.2021 14:00

Physics, 20.06.2021 14:00

History, 20.06.2021 14:00



Biology, 20.06.2021 14:00


Mathematics, 20.06.2021 14:00

Biology, 20.06.2021 14:00


Mathematics, 20.06.2021 14:00



Social Studies, 20.06.2021 14:00


Spanish, 20.06.2021 14:00

English, 20.06.2021 14:00

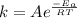 , where k is the rate constant, A is the frequency factor,
, where k is the rate constant, A is the frequency factor, 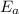 is the activation energy, R is the gas constant, and T is the temperature in Kelvins.
is the activation energy, R is the gas constant, and T is the temperature in Kelvins.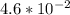 . So:
. So: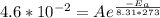
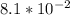 . So:
. So: