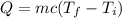4 points
221.7 g sample of water that begins at 25.0 degrees Celsius is heated with 3560.6 cal...

Chemistry, 22.05.2020 22:02 glowbaby123
4 points
221.7 g sample of water that begins at 25.0 degrees Celsius is heated with 3560.6 calories of heat. What is the final temperature of the w
Type your answer.
Previous

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
Questions

Computers and Technology, 04.08.2019 01:00

History, 04.08.2019 01:00


Mathematics, 04.08.2019 01:00



Mathematics, 04.08.2019 01:00

History, 04.08.2019 01:00


Biology, 04.08.2019 01:00

Mathematics, 04.08.2019 01:00

Mathematics, 04.08.2019 01:00

Mathematics, 04.08.2019 01:00

Mathematics, 04.08.2019 01:00

Mathematics, 04.08.2019 01:00

Physics, 04.08.2019 01:00



Biology, 04.08.2019 01:00