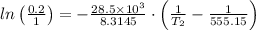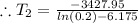The normal boiling point of a liquid is 282 °C. At what temperature (in
°C) would the vapor pr...

Chemistry, 22.05.2020 22:59 veronica25681
The normal boiling point of a liquid is 282 °C. At what temperature (in
°C) would the vapor pressure be 0.2 atm? (AHvap = 28.5 kJ/mol)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
Questions

Mathematics, 17.02.2021 01:00


Advanced Placement (AP), 17.02.2021 01:00

History, 17.02.2021 01:00

Mathematics, 17.02.2021 01:00


Mathematics, 17.02.2021 01:00


Mathematics, 17.02.2021 01:00


English, 17.02.2021 01:00


Mathematics, 17.02.2021 01:00


English, 17.02.2021 01:00


Health, 17.02.2021 01:00



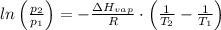
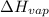 = The heat of vaporization = 28.5 kJ/mol
= The heat of vaporization = 28.5 kJ/mol