HW) Aspirin Poisoning
When your body fluids contain too much acid, it’s known as acidosi...

HW) Aspirin Poisoning
When your body fluids contain too much acid, it’s known as acidosis. Acidosis occurs when your kidneys and lungs can’t keep your body’s pH in balance. Many of the body’s processes produce acid. Your lungs and kidneys can usually compensate for slight pH imbalances, but problems with these organs can lead to excess acid accumulating in your body.
The acidity of your blood is measured by determining its pH. The pH of your blood should be around 7.4. According to the American Association for Clinical Chemistry (AACC), acidosis is characterized by a pH of 7.35 or lower. Alkalosis (too much base) characterized by a pH level of 7.45 or higher. While seemingly slight, these numerical differences can be serious.
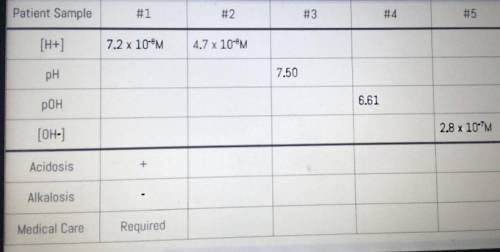
YOUR TASK: To determine whether or not the patient requires immediate medical care based on blood sample data.
The chart below represents blood sample data received from patients reporting aspirin overdose. Complete each patient’s chart and determine whether or not immediate medical care is necessary for metabolic acidosis and/or respiratory alkalosis. According to the AACC, normal blood pH ranges between 7.35 and 7.45. As evidence, you must show all your work.
Pls give me the answers
I’ll give brainliest answer
Please get them correct

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Questions


History, 24.01.2020 20:31


English, 24.01.2020 20:31




History, 24.01.2020 20:31

Computers and Technology, 24.01.2020 20:31




Mathematics, 24.01.2020 20:31


World Languages, 24.01.2020 20:31


Social Studies, 24.01.2020 20:31

English, 24.01.2020 20:31

History, 24.01.2020 20:31

English, 24.01.2020 20:31



