
Chemistry, 23.05.2020 19:03 fatherbamboo
How many moles of barium hydroxide can be produced from the reaction of 4.234 moles of barium with 8.902 moles of water according to the following reaction? Ba + 2 H2O Ba(OH)2 + H2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
How many moles of barium hydroxide can be produced from the reaction of 4.234 moles of barium with 8...
Questions

Mathematics, 25.09.2021 14:00

History, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00


History, 25.09.2021 14:00

English, 25.09.2021 14:00



Social Studies, 25.09.2021 14:00

Health, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00




Mathematics, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00



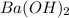 will be produced from the given masses of both reactants.
will be produced from the given masses of both reactants.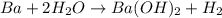
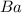 require = 2 moles of
require = 2 moles of 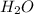
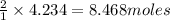 of
of 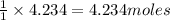 of
of 

