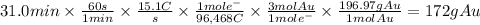Metal plating is done by passing a current through a metal solution. For example, an item can become gold plated by attaching the item to a power source and submerging it into an Au³⁺ solution. The item itself serves as the cathode, at which the Au³⁺ ions are reduced to Au(s). A piece of solid gold is used as the anode and is also connected to the power source, thus completing the circuit. What mass of gold is produced when 15.1 A of current are passed through a gold solution for 31.0 min?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

You know the right answer?
Metal plating is done by passing a current through a metal solution. For example, an item can become...
Questions


Computers and Technology, 22.07.2019 11:00

Computers and Technology, 22.07.2019 11:00

German, 22.07.2019 11:00

English, 22.07.2019 11:00

Arts, 22.07.2019 11:00



History, 22.07.2019 11:00

Computers and Technology, 22.07.2019 11:00