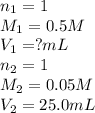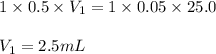How much of 0.5 M HNO3 is necessary to titrate 25.0 mL of 0.05 M KOH
solution to the endpoint?...

Chemistry, 25.05.2020 05:57 mikewalton566
How much of 0.5 M HNO3 is necessary to titrate 25.0 mL of 0.05 M KOH
solution to the endpoint?
Show all work using the titrations formula.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Questions


Biology, 04.03.2021 01:50



Mathematics, 04.03.2021 01:50


Computers and Technology, 04.03.2021 01:50

Mathematics, 04.03.2021 01:50


Mathematics, 04.03.2021 01:50




Mathematics, 04.03.2021 01:50



Mathematics, 04.03.2021 01:50


Mathematics, 04.03.2021 01:50

Mathematics, 04.03.2021 01:50

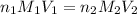
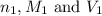 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 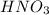
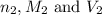 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.