- Given the following experimental data, use the method of initial
rates to determine the rate...

Chemistry, 26.05.2020 01:59 lydiadmanautou04
- Given the following experimental data, use the method of initial
rates to determine the rate law for the reaction aA + bB --> products.
Hint: Any number to the zero power equals one. For example,
(0.22) 1 and (55.6)° = 1.
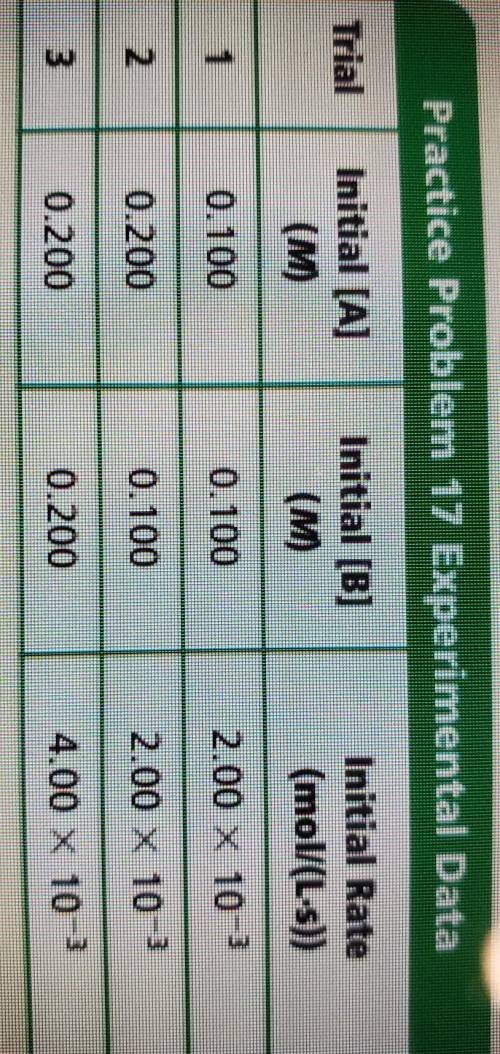

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
Questions

Biology, 17.10.2019 13:30

Mathematics, 17.10.2019 13:30






Mathematics, 17.10.2019 13:30

Mathematics, 17.10.2019 13:30


Mathematics, 17.10.2019 13:30

History, 17.10.2019 13:30

Mathematics, 17.10.2019 13:30


Mathematics, 17.10.2019 13:30

Mathematics, 17.10.2019 13:30

Social Studies, 17.10.2019 13:30



History, 17.10.2019 13:30



