1. How many molecules of barium hydroxide, Ba(OH)2 are there in 21.9 grams of
barium hydroxide...

Chemistry, 26.05.2020 05:57 ayleenmorar
1. How many molecules of barium hydroxide, Ba(OH)2 are there in 21.9 grams of
barium hydroxide?
A. 6.022 x 1023 molecules Ba(OH)2
B. 7.7 x 1022 molecules Ba(OH)2
C. 6.23 x 1021 molecules Ba(OH)2
D. 5.93 x 1021 molecules Ba(OH)2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Questions

Geography, 02.12.2020 03:00


History, 02.12.2020 03:00



English, 02.12.2020 03:00

Mathematics, 02.12.2020 03:00



English, 02.12.2020 03:00

Geography, 02.12.2020 03:00

Mathematics, 02.12.2020 03:00

Mathematics, 02.12.2020 03:00

Mathematics, 02.12.2020 03:00



Mathematics, 02.12.2020 03:00


Mathematics, 02.12.2020 03:00

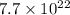 molecules of
molecules of 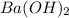
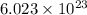 of particles.
of particles.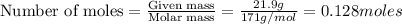
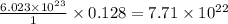 molecules
molecules

