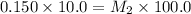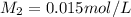1. 90.0 mL of distilled water is added to a 10.0mL sample of 0.150mol/L sodium
chloride. The r...

Chemistry, 27.05.2020 02:58 xaviiaquino3378
1. 90.0 mL of distilled water is added to a 10.0mL sample of 0.150mol/L sodium
chloride. The resulting solution can be described as more
a. dilute
b. concentrated
c. Acidic
d. basic

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2
You know the right answer?
Questions


Mathematics, 14.06.2021 16:00

Chemistry, 14.06.2021 16:00













Mathematics, 14.06.2021 16:00

Mathematics, 14.06.2021 16:00


Business, 14.06.2021 16:00

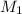 =concentration of stock solution = 0.150 mol/L
=concentration of stock solution = 0.150 mol/L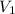 = volume of stock solution = 10.0 ml
= volume of stock solution = 10.0 ml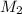 = concentration of dilute solution = ?
= concentration of dilute solution = ?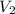 = volume of dilute solution = (10.0+90.0) ml = 100.0 ml
= volume of dilute solution = (10.0+90.0) ml = 100.0 ml