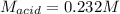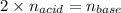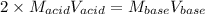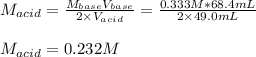Chemistry, 27.05.2020 03:59 koreanrice101
What is the molarity of a solution of sulfuric acid if 49.0 mL of it are neutralized by a titration
of 68.4 mL of 0.333 M sodium hydroxide solution? (Round to the correct number of sig figs
and include your units!)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
What is the molarity of a solution of sulfuric acid if 49.0 mL of it are neutralized by a titration<...
Questions


Social Studies, 04.08.2019 04:30







Mathematics, 04.08.2019 04:30