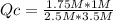Chemistry, 27.05.2020 11:57 antionette1
HCl (g) + NaOH (aq) <-> NaCl (aq) + H2O (l). What is the Q value for the equation and which direction will the reaction shift given that K = 0.5? , [HCl] = 2.5 M, [NaOH] = 3.5 M, [NaCl] = 1.75 M, [H2O] = 1 M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
HCl (g) + NaOH (aq) <-> NaCl (aq) + H2O (l). What is the Q value for the equation and which di...
Questions


Mathematics, 19.05.2021 18:20






Mathematics, 19.05.2021 18:20



Mathematics, 19.05.2021 18:20

Arts, 19.05.2021 18:20



English, 19.05.2021 18:20


English, 19.05.2021 18:20

Engineering, 19.05.2021 18:20

Mathematics, 19.05.2021 18:20

![Qc=\frac{[C]^{c}*[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0667/0235/1eb27.png)
![Qc=\frac{[NaCl]*[H_{2}O] }{[HCl]*[NaOH]}](/tpl/images/0667/0235/1a295.png)