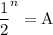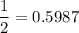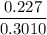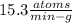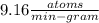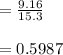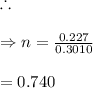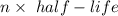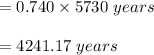In living organisms, C-14 atoms disintegrate at a rate of 15.3 atoms per minute per gram of carbon. A charcoal sample from an archaeological site has a C-14 disintegration rate of 9.16 atoms per minute per gram of carbon. Estimate the age of this sample in years. The half-life of C-14 is 5730 years. (enter only the number of years in standard notation, not the unit years)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
In living organisms, C-14 atoms disintegrate at a rate of 15.3 atoms per minute per gram of carbon....
Questions

Mathematics, 30.06.2019 16:00



Mathematics, 30.06.2019 16:00

History, 30.06.2019 16:00


History, 30.06.2019 16:00

Mathematics, 30.06.2019 16:00

Mathematics, 30.06.2019 16:00

Mathematics, 30.06.2019 16:00


Biology, 30.06.2019 16:00


Mathematics, 30.06.2019 16:00

Computers and Technology, 30.06.2019 16:00

Social Studies, 30.06.2019 16:00


Mathematics, 30.06.2019 16:00


Physics, 30.06.2019 16:00

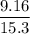 = 0.5987
= 0.5987