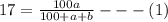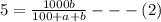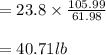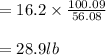Soda and lime are added to a glass batch in the form of soda ash (Na2CO3 ) and lime-stone (CaCO3 ). During heating, these two ingredients decompose to give off carbon dioxide (CO2 ), the resulting products being soda and lime. Compute the weight of soda ash and limestone that must be added to 125 lbm of quartz (SiO2 ) to yield a glass of composition 78 wt% SiO2 , 17 wt% Na2O, and 5 wt% CaO.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
Soda and lime are added to a glass batch in the form of soda ash (Na2CO3 ) and lime-stone (CaCO3 )....
Questions

Mathematics, 06.04.2020 01:58



Mathematics, 06.04.2020 01:58

Computers and Technology, 06.04.2020 01:58

Business, 06.04.2020 01:58

Computers and Technology, 06.04.2020 01:58

Mathematics, 06.04.2020 01:58

Physics, 06.04.2020 01:59


Mathematics, 06.04.2020 01:59

Geography, 06.04.2020 01:59

History, 06.04.2020 01:59



Mathematics, 06.04.2020 01:59