5. A standardized mass was known to weigh exactly 5.0000g. It was weighed on a newly
acquired,...

Chemistry, 28.05.2020 01:01 floreschachi2873
5. A standardized mass was known to weigh exactly 5.0000g. It was weighed on a newly
acquired, uncalibrated balance as 5.001g. Calculate the absolute error and the percent error.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1


Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Questions

Mathematics, 14.01.2021 07:20



Engineering, 14.01.2021 07:20

History, 14.01.2021 07:20

Mathematics, 14.01.2021 07:20

History, 14.01.2021 07:20


Mathematics, 14.01.2021 07:20


History, 14.01.2021 07:20


English, 14.01.2021 07:20




Mathematics, 14.01.2021 07:20

Mathematics, 14.01.2021 07:20


Mathematics, 14.01.2021 07:20

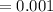
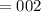 5
5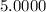 grams
grams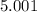 grams
grams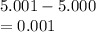
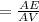
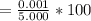
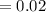 %
%

