
Chemistry, 28.05.2020 03:57 arianawelsh123l
"A gas turbine receives a mixture having the following molar analysis: 10% CO2, 19% H2O, 71% N2, at 720 K, 0.35 MPa and a volumetric flow rate of 3.2 m3 /s. The mixture exits the turbine at 380 K, 0.11 MPa. For adiabatic operation with negligible kinetic and potential energy effects, determine the power developed at steady state, in kW." NOTE: the process is NOT isentropic.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
"A gas turbine receives a mixture having the following molar analysis: 10% CO2, 19% H2O, 71% N2, at...
Questions


Chemistry, 29.08.2019 20:30

Biology, 29.08.2019 20:30

History, 29.08.2019 20:30

Mathematics, 29.08.2019 20:30

Mathematics, 29.08.2019 20:30





Physics, 29.08.2019 20:30

English, 29.08.2019 20:30

Spanish, 29.08.2019 20:30

Geography, 29.08.2019 20:30


Mathematics, 29.08.2019 20:30


Mathematics, 29.08.2019 20:30

English, 29.08.2019 20:30

Health, 29.08.2019 20:30

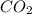 =0.1
=0.1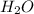 = O.19
= O.19
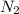 =0.71
=0.71
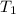 ) mixture receives from turbine =720K
) mixture receives from turbine =720K
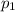 ) mixture receives from turbine =0.35 Mpa
) mixture receives from turbine =0.35 Mpa
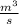
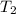 ) = 380K
) = 380K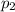 )= 0.11 Mpa
)= 0.11 Mpa
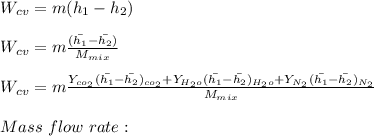
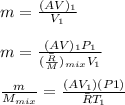
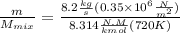
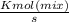
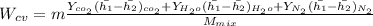
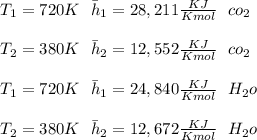
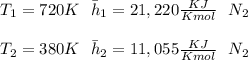
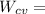 2074.2 KW
2074.2 KW

