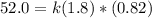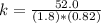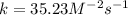Chemistry, 27.05.2020 19:06 FriendlyDude640
Rate of a certain reaction is given by the following rate law: rate Use this information to answer the questions below. What is the reaction order in H2? What is the reaction order in I2? What is overall reaction order? At a certain concentration of H2 and I2, the initial rate of reaction is 2.0 x 104 M / s. What would the initial rate of the reaction be if the concentration of H2 were doubled? Round your answer to significant digits. The rate of the reaction is measured to be 52.0 M / s when [H2] = 1.8 M and [I2] = 0.82 M. Calculate the value of the rate constant. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 13:30
If a fast moving car making a loud noise approaches and moves past the person what will happen as the distance between the two increases?
Answers: 1

Chemistry, 23.06.2019 14:30
The first supersonic flight was in 1947. it was just above the speed of sound. which altitude would you expect captain yeager to have used for his flight
Answers: 3

Chemistry, 23.06.2019 17:30
Describe the type of force which exists between particles in an ideal gas. explain why this type of force exists between the particles.
Answers: 3
You know the right answer?
Rate of a certain reaction is given by the following rate law: rate Use this information to answer t...
Questions

Mathematics, 02.01.2022 18:50

Mathematics, 02.01.2022 18:50


Biology, 02.01.2022 19:00


Computers and Technology, 02.01.2022 19:00

English, 02.01.2022 19:00

Mathematics, 02.01.2022 19:00

Mathematics, 02.01.2022 19:00

Chemistry, 02.01.2022 19:00


Computers and Technology, 02.01.2022 19:00

History, 02.01.2022 19:00

Mathematics, 02.01.2022 19:00

Mathematics, 02.01.2022 19:00

Mathematics, 02.01.2022 19:00

History, 02.01.2022 19:00

English, 02.01.2022 19:00

Social Studies, 02.01.2022 19:00

![rate = k [H_2][I_2]](/tpl/images/0667/2967/0cf2e.png)
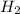 ?
? 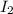 ?
? 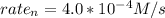
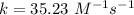
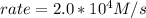
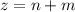
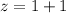
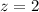
![2.0*10^{4} = k [H_2] [I_2] ---(1)](/tpl/images/0667/2967/d17d0.png)
![k = \frac{2.0*10^{4}}{[H_2] [I_2] }](/tpl/images/0667/2967/c78a9.png)
![rate = k [2H_2] [I_2] ----(2)](/tpl/images/0667/2967/37402.png)
![k = \frac{rate_n }{ [2H_2] [I_2]}](/tpl/images/0667/2967/3b211.png)
![\frac{2.0*10^{4}}{[H_2] [I_2] } = \frac{rate_n }{ [2H_2] [I_2]}](/tpl/images/0667/2967/1c986.png)
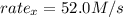
![[H_2] = 1.8 M](/tpl/images/0667/2967/35dd9.png)
![[I_2] = 0.82 \ M](/tpl/images/0667/2967/8a61e.png)