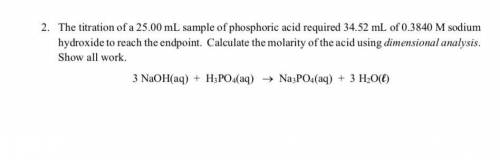
Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
PLEASE PLEASEE HELP ASAP THANK YOU
...
...
Questions

History, 12.07.2019 01:10

Biology, 12.07.2019 01:10

Biology, 12.07.2019 01:10

Biology, 12.07.2019 01:10

Biology, 12.07.2019 01:10

English, 12.07.2019 01:20



Mathematics, 12.07.2019 01:20

Mathematics, 12.07.2019 01:20




Mathematics, 12.07.2019 01:20



Mathematics, 12.07.2019 01:20

Chemistry, 12.07.2019 01:20

Mathematics, 12.07.2019 01:20