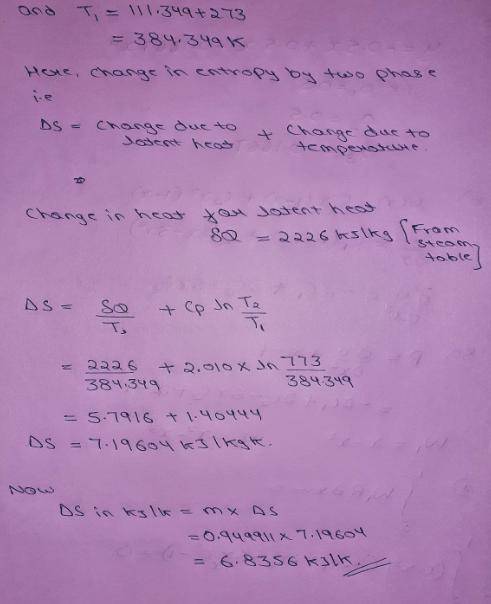Chemistry, 28.05.2020 19:01 donaldplawlerp5cctt
An insulated piston-cylinder device contains 1 L of saturated liquid water at pressure of 150 kPa. An electric resistance heater inside the cylinder is now turned on, and 2869.47 kJ of heat is transferred to the water. The inside H2O pressure maintains constant at 150 kPa during the process. Determine: the entropy change of the water during this heating process, in kJ/K.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
An insulated piston-cylinder device contains 1 L of saturated liquid water at pressure of 150 kPa. A...
Questions





Social Studies, 26.02.2021 19:40


Mathematics, 26.02.2021 19:40



Mathematics, 26.02.2021 19:40

Social Studies, 26.02.2021 19:40


Mathematics, 26.02.2021 19:40

Biology, 26.02.2021 19:40




English, 26.02.2021 19:40


Mathematics, 26.02.2021 19:40