
Chemistry, 28.05.2020 23:03 saneayahsimmons
Given the equilibrium: A+B <--> C. The greatest concentration of C would be produced if the equilibrium constant of the reaction is equal to
a. 1x10^-9
b. 1x10^9
c. 1x10^-3
d. 1x10^3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3


You know the right answer?
Given the equilibrium: A+B <--> C. The greatest concentration of C would be produced if the eq...
Questions

Mathematics, 26.05.2020 18:59

Mathematics, 26.05.2020 18:59


Mathematics, 26.05.2020 18:59


Mathematics, 26.05.2020 18:59

Computers and Technology, 26.05.2020 18:59




Mathematics, 26.05.2020 19:00

English, 26.05.2020 19:00


Mathematics, 26.05.2020 19:00




Biology, 26.05.2020 19:00

History, 26.05.2020 19:00

Biology, 26.05.2020 19:00

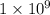
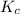
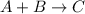
![K_c=\frac{[C]}{[A][B]}](/tpl/images/0669/3501/66d11.png)


