
Chemistry, 29.05.2020 00:58 NickWinston261
What would be the pH at the equivalence point of a 0.01M solution of strong acid titrated using 45mL of 0.02Mof a strong base? *

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 3

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2
You know the right answer?
What would be the pH at the equivalence point of a 0.01M solution of strong acid titrated using 45mL...
Questions

Chemistry, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50



Chemistry, 03.11.2020 18:50









Chemistry, 03.11.2020 18:50


Mathematics, 03.11.2020 18:50



Mathematics, 03.11.2020 18:50

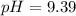
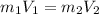
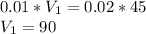
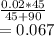
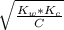
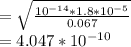
![pH = -log [H^+]](/tpl/images/0669/5500/9b8d2.png)


