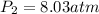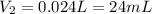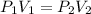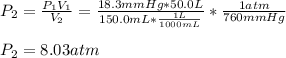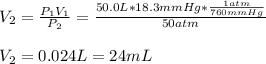Chemistry, 29.05.2020 03:02 Franklyn3834
3. A 50.0 L sample of gas collected in the upper atmosphere at a pressure of 18.3 mmHg is compressed into a 150.0 mL container at the same temperature.
a. What is the new pressure, in atm?
b. To what volume would the original sample have had to be compressed to exert a pressure of 10.0 atm?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 12:50
Which of these describes the rate of this chemical reaction? h2 + cl2 → 2 hcl a. an increase in the concentration of hcl and h2 with time b. an increase in the concentration of hcl with time c. an increase in h2 and cl2 with time d. a decrease in hcl and cl2 with time
Answers: 1
You know the right answer?
3. A 50.0 L sample of gas collected in the upper atmosphere at a pressure of 18.3 mmHg is compressed...
Questions


Mathematics, 05.05.2020 07:15



Mathematics, 05.05.2020 07:15

Chemistry, 05.05.2020 07:15

Mathematics, 05.05.2020 07:15

Mathematics, 05.05.2020 07:15


Geography, 05.05.2020 07:15

Mathematics, 05.05.2020 07:15

Mathematics, 05.05.2020 07:15

History, 05.05.2020 07:15

Mathematics, 05.05.2020 07:15


Mathematics, 05.05.2020 07:15

Health, 05.05.2020 07:15



Mathematics, 05.05.2020 07:15