
Chemistry, 29.05.2020 05:00 Coltong121
One millimole of Ni(NO3)2 dissolves in 240.0 mL of a solution that is 0.500 M in ammonia. The formation constant of Ni(NH3)62+ is 5.5×108. What is the initial concentration of Ni(NO3)2 in the solution? What is the equilibrium concentration of Ni2+(aq ) in the solution?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1

You know the right answer?
One millimole of Ni(NO3)2 dissolves in 240.0 mL of a solution that is 0.500 M in ammonia. The format...
Questions

Mathematics, 02.02.2020 04:44

Mathematics, 02.02.2020 04:44

Mathematics, 02.02.2020 04:44


Arts, 02.02.2020 04:44



Mathematics, 02.02.2020 04:44

English, 02.02.2020 04:44


Mathematics, 02.02.2020 04:44


Mathematics, 02.02.2020 04:44



Mathematics, 02.02.2020 04:44

Physics, 02.02.2020 04:44



![[Ni^{2+}]_0=0.0042M](/tpl/images/0669/8824/46776.png)
![[Ni^{2+}]_{eq}=0M](/tpl/images/0669/8824/028c2.png)
![Ni^{+2}+6NH_3\rightleftharpoons [Ni(NH_3)_6]^{2+}](/tpl/images/0669/8824/85f00.png)
![Kf=\frac{[Ni(NH_3)_6]^{2+}}{[NH_3]^6[Ni^{+2}]}](/tpl/images/0669/8824/f8603.png)
![[Ni^{2+}]_0=\frac{0.001mol}{0.240L} =0.0042M](/tpl/images/0669/8824/ca35e.png)
 due to the reaction extent:
due to the reaction extent: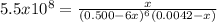
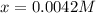
![[Ni^{2+}]_{eq}=0.0042M-x=0.0042M-0.0042M=0](/tpl/images/0669/8824/b9bd2.png)


