
Chemistry, 29.05.2020 06:02 angelZ3947
The first two steps in the industrial synthesis of nitric acid produce nitrogen dioxide from ammonia: The net reaction is: Write an equation that gives the overall equilibrium constant in terms of the equilibrium constants and . If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
The first two steps in the industrial synthesis of nitric acid produce nitrogen dioxide from ammonia...
Questions





Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

English, 13.10.2020 15:01

History, 13.10.2020 15:01



Social Studies, 13.10.2020 15:01


Mathematics, 13.10.2020 15:01


Mathematics, 13.10.2020 15:01


Biology, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

Biology, 13.10.2020 15:01

History, 13.10.2020 15:01

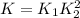
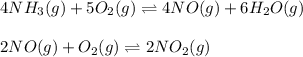
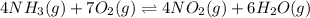
![K_1=\frac{[NO]^4[H_2O]^6}{[NH_3]^4[O_2]^5}\\ \\K_2=\frac{[NO_2]^2}{[NO]^2[O_2]}](/tpl/images/0669/9771/004ed.png)
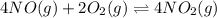
![K_2^{new}=\frac{[NO_2]^4}{[NO]^4[O_2]^2}](/tpl/images/0669/9771/b0ba1.png)
![K_1*K_2^2=\frac{[NO]^4[H_2O]^6}{[NH_3]^4[O_2]^5}*\frac{[NO_2]^4}{[NO]^4[O_2]^2}=\frac{[H_2O]^6[NO_2]^4}{[NH_3]^4[O_2]^7}](/tpl/images/0669/9771/5162f.png)
![K=\frac{[H_2O]^6[NO_2]^4}{[NH_3]^4[O_2]^7}](/tpl/images/0669/9771/dc773.png)


