
Chemistry, 29.05.2020 19:03 emilyanneK2540
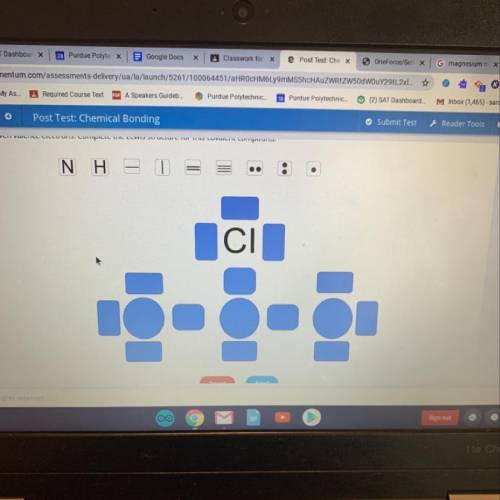
Drag the tiles to the correct locations. Each tile can be used more than once, but not all tiles will be used. Some locations will remain empty.
Chloramine has the chemical formula NH, CI. Nitrogen has five valence electrons, each hydrogen has one valence electron, and chlorine has
seven valence electrons. Complete the Lewis structure for this covalent compound.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3


Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Drag the tiles to the correct locations. Each tile can be used more than once, but not all tiles wil...
Questions



Mathematics, 12.02.2020 23:56

Mathematics, 12.02.2020 23:56

Mathematics, 12.02.2020 23:56




Business, 12.02.2020 23:57

Social Studies, 12.02.2020 23:57




Geography, 12.02.2020 23:57

History, 12.02.2020 23:57



History, 12.02.2020 23:57




