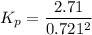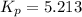For the reaction: 2 A (g) + B (s)= 2 C(s) + D (g)
At 298 K in a 10.0 L vessel, the equilibrium...

Chemistry, 29.05.2020 19:03 amberskids2
For the reaction: 2 A (g) + B (s)= 2 C(s) + D (g)
At 298 K in a 10.0 L vessel, the equilibrium values are as follows: 0.721 atm of A, 4.18 mol of B, 6.25 mol of C, and 2.71 atm of D. What is the value of the equilibrium constant?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Questions


Social Studies, 31.08.2019 21:50


Biology, 31.08.2019 21:50

Social Studies, 31.08.2019 21:50



Biology, 31.08.2019 21:50

Computers and Technology, 31.08.2019 21:50

Mathematics, 31.08.2019 21:50

English, 31.08.2019 21:50


English, 31.08.2019 21:50


SAT, 31.08.2019 21:50


Chemistry, 31.08.2019 21:50



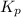 (equilibrium constant) is referred to as the partial pressure of product divided by the partial pressure of reactant with each pressure term raised to power that is equal to its stoichiometric coefficient in balanced equation
.
(equilibrium constant) is referred to as the partial pressure of product divided by the partial pressure of reactant with each pressure term raised to power that is equal to its stoichiometric coefficient in balanced equation
.![K_p = \dfrac{[D]}{[A]^2}](/tpl/images/0670/4630/dcbe2.png)