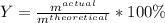In the reaction between CO and Fe3O4, the theoretical yield in an experiment is calculated to be 47.2 g Fe. When a chemistry student carries out the experiment, the actual yield is 29.9 g Fe. Calculate the percentage yield. % Please show all work.
a. .633%
b 157%
c 63.3%
d .157%

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Why should the scientific method be used to answer a question? a. it provides a way to test an idea without any bias. b. it provides a way to test a hypothesis. c. it provides a way to ensure all hypotheses are proven correct. d. it provides a way to quickly turn a hypothesis into a scientific theory.
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
In the reaction between CO and Fe3O4, the theoretical yield in an experiment is calculated to be 47....
Questions




Business, 03.08.2019 07:30

Business, 03.08.2019 07:30


Computers and Technology, 03.08.2019 07:30


Chemistry, 03.08.2019 07:30



Business, 03.08.2019 07:30


Biology, 03.08.2019 07:30


History, 03.08.2019 07:30


Mathematics, 03.08.2019 07:30

Mathematics, 03.08.2019 07:30