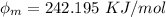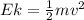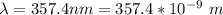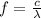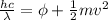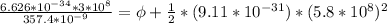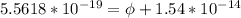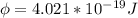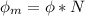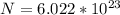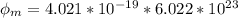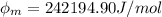Chemistry, 29.05.2020 09:57 lilyrockstarmag
In order to comply with the requirement that energy be conserved, Einstein showed in the photoelectric effect that the energy of a photon (h) absorbed by a metal is the sum of the work function (), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron: h = + Ek. When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is (in kJ/mol) of potassium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
In order to comply with the requirement that energy be conserved, Einstein showed in the photoelectr...
Questions



Biology, 02.08.2019 00:30


History, 02.08.2019 00:30

Mathematics, 02.08.2019 00:30




English, 02.08.2019 00:30

History, 02.08.2019 00:30

Health, 02.08.2019 00:30

English, 02.08.2019 00:30



English, 02.08.2019 00:30

Biology, 02.08.2019 00:30



Biology, 02.08.2019 00:30

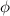 ), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron:
), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron: 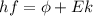 . When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is work function (in kJ/mol) of potassium?
. When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is work function (in kJ/mol) of potassium?