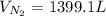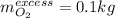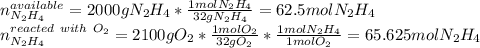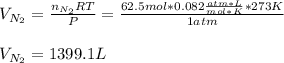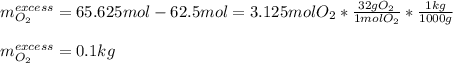Chemistry, 29.05.2020 22:57 bvargas786p7aa8y
Hydrazine (N2H4) is used as rocket fuel. It reacts with oxygen to form nitrogen and water.
N2H4(l) + O2(g) N2(g) + 2H2O(g)
a. How many liters of N2 (at STP) form when 2.0 kg N2H4 reacts with 2.1 kg O2?
b. How many grams of the excess reagent remain after the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

You know the right answer?
Hydrazine (N2H4) is used as rocket fuel. It reacts with oxygen to form nitrogen and water.
Questions

History, 11.11.2019 07:31

Mathematics, 11.11.2019 07:31




Mathematics, 11.11.2019 07:31








History, 11.11.2019 07:31

Mathematics, 11.11.2019 07:31



Mathematics, 11.11.2019 07:31


English, 11.11.2019 07:31