
Chemistry, 29.05.2020 22:58 yulimariu27
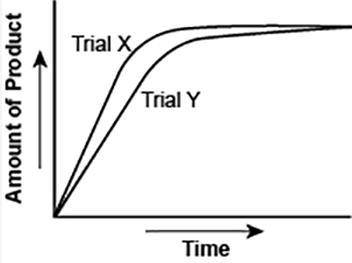
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A) Trial X, because the final volume of product formed is lower than Trial Y.
B) Trial X, because this reaction was initially fast and later stopped completely.
C) Trial Y, because the reaction was initially slow and later stopped completely.
D) Trial Y, because the volume of product formed per unit time is lower than Trial X.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions



Computers and Technology, 06.07.2019 01:20


Computers and Technology, 06.07.2019 01:20


Computers and Technology, 06.07.2019 01:20



English, 06.07.2019 01:20


Computers and Technology, 06.07.2019 01:20





French, 06.07.2019 01:20






