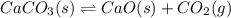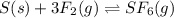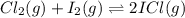Chemistry, 29.05.2020 13:57 lanakay2006
Changes in pressure can have a large effect on equilibrium systems containing gaseous components.
1. changing the concentration of gaseous components
2. adding an inert gas has no effect since the gas does not take part in the reaction, all partial pressures stay the same
3.changing the volume of the reaction vessel. This will cause a shift in the equilibrium position if the number of moles of gas is different on the reactant and product side (so Δn = n products - n reactants)
How would you change the volume for each of the following reactions to increase the yield of the product(s)?1. CaCO3(s) ⇋ CaO(s) + CO2(g) (increase, decrease, no change)2. S(s) + 3F2(g) ⇋ SF6(g) (increase, decrease, no change)3. Cl2(g) + I2(g) ⇋ 2ICl(g) (increase, decrease, no change)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Changes in pressure can have a large effect on equilibrium systems containing gaseous components.
Questions


Mathematics, 18.04.2021 23:20


Mathematics, 18.04.2021 23:20


Mathematics, 18.04.2021 23:20




Mathematics, 18.04.2021 23:20

English, 18.04.2021 23:20

Mathematics, 18.04.2021 23:20

Chemistry, 18.04.2021 23:20

Mathematics, 18.04.2021 23:20



English, 18.04.2021 23:20


History, 18.04.2021 23:20

Mathematics, 18.04.2021 23:20